AI tutor
Welcome to Bytelearn!
Let’s check out your problem:
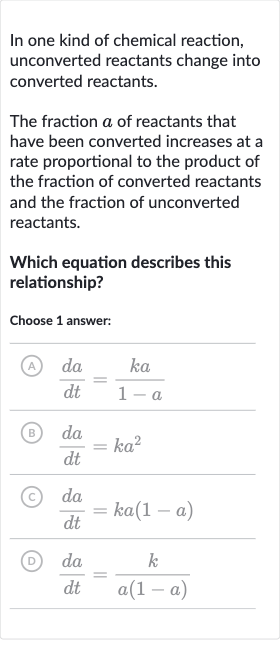
In one kind of chemical reaction, unconverted reactants change into converted reactants.The fraction of reactants that have been converted increases at a rate proportional to the product of the fraction of converted reactants and the fraction of unconverted reactants.Which equation describes this relationship?Choose answer:(A) (B) (C) (D)
Full solution
Q. In one kind of chemical reaction, unconverted reactants change into converted reactants.The fraction of reactants that have been converted increases at a rate proportional to the product of the fraction of converted reactants and the fraction of unconverted reactants.Which equation describes this relationship?Choose answer:(A) (B) (C) (D)
- Denote Fraction of Reactants: Let's denote the fraction of reactants that have been converted by . According to the problem, the rate of change of with respect to time , which is , is proportional to the product of the fraction of converted reactants and the fraction of unconverted reactants . The constant of proportionality is . Therefore, the equation that describes this relationship is .
- Rate of Change Equation: We can now compare the given options with our derived equation. Option (A) does not match because it suggests the rate is inversely proportional to the fraction of unconverted reactants, which is not what the problem states.
- Comparison with Options: Option (B) suggests the rate is proportional to the square of the fraction of converted reactants, which again is not what the problem states.
- Option (A) Analysis: Option (C) matches our derived equation exactly, indicating that the rate of conversion is proportional to both the fraction of converted reactants and the fraction of unconverted reactants.
- Option (B) Analysis: Option (D) suggests the rate is inversely proportional to the product of the fraction of converted and unconverted reactants, which is incorrect according to the problem statement.
More problems from Write two-variable inequalities: word problems
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
