AI tutor
Welcome to Bytelearn!
Let’s check out your problem:
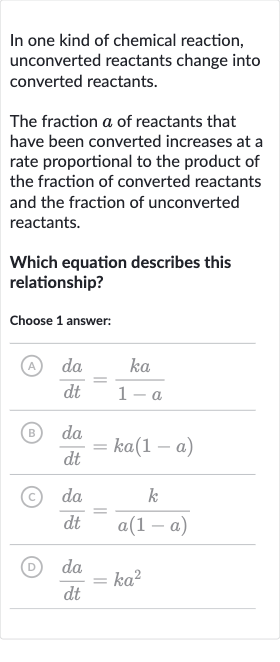
In one kind of chemical reaction, unconverted reactants change into converted reactants.The fraction of reactants that have been converted increases at a rate proportional to the product of the fraction of converted reactants and the fraction of unconverted reactants.Which equation describes this relationship?Choose answer:(A) (B) (c) (D)
Full solution
Q. In one kind of chemical reaction, unconverted reactants change into converted reactants.The fraction of reactants that have been converted increases at a rate proportional to the product of the fraction of converted reactants and the fraction of unconverted reactants.Which equation describes this relationship?Choose answer:(A) (B) (c) (D)
- Define Fraction Conversion: Let's denote the fraction of reactants that have been converted by . According to the problem, the rate of change of with respect to time , denoted as , is proportional to the product of the fraction of converted reactants and the fraction of unconverted reactants . The constant of proportionality is .
- Rate of Change Equation: The equation that describes this relationship should therefore be , where is the constant of proportionality.
- Match with Given Choices: Looking at the given choices, we can see that option (B) matches the equation we derived in the previous step.
More problems from Write two-variable inequalities: word problems
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
QuestionGet tutor help
