AI tutor
Welcome to Bytelearn!
Let’s check out your problem:
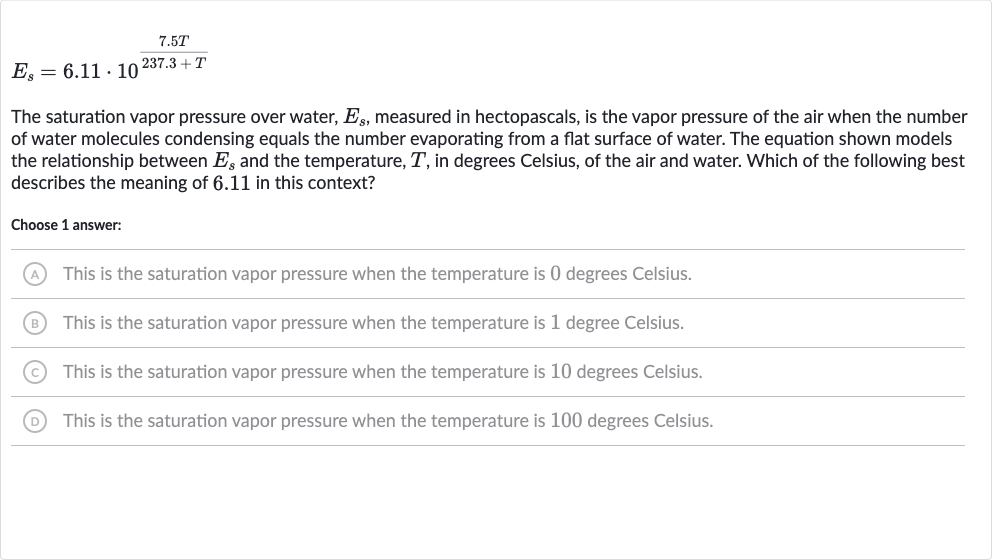
The saturation vapor pressure over water, , measured in hectopascals, is the vapor pressure of the air when the number of water molecules condensing equals the number evaporating from a flat surface of water. The equation shown models the relationship between and the temperature, , in degrees Celsius, of the air and water. Which of the following best describes the meaning of . in this context?Choose answer:(A) This is the saturation vapor pressure when the temperature is degrees Celsius.(B) This is the saturation vapor pressure when the temperature is degree Celsius.(C) This is the saturation vapor pressure when the temperature is degrees Celsius.(D) This is the saturation vapor pressure when the temperature is degrees Celsius.
Full solution
Q. The saturation vapor pressure over water, , measured in hectopascals, is the vapor pressure of the air when the number of water molecules condensing equals the number evaporating from a flat surface of water. The equation shown models the relationship between and the temperature, , in degrees Celsius, of the air and water. Which of the following best describes the meaning of . in this context?Choose answer:(A) This is the saturation vapor pressure when the temperature is degrees Celsius.(B) This is the saturation vapor pressure when the temperature is degree Celsius.(C) This is the saturation vapor pressure when the temperature is degrees Celsius.(D) This is the saturation vapor pressure when the temperature is degrees Celsius.
- Understand Equation and Constant: Understand the equation and identify the constant.The equation models the relationship between the saturation vapor pressure and the temperature in degrees Celsius. The constant is a part of this equation, and we need to determine its significance.
- Analyze Role of : Analyze the equation to determine the role of . To understand the meaning of , we need to consider the equation at a specific temperature. The most logical temperature to consider is degrees Celsius, as it is a standard reference point for many scientific measurements.
- Substitute : Substitute into the equation to see the effect on . If we substitute into the equation, we get . This simplifies to , since any number to the power of is .
- Calculate Vapor Pressure: Calculate the saturation vapor pressure at degrees Celsius.Now, we calculate with : . This means that the saturation vapor pressure at degrees Celsius is hectopascals.
